- Modern drug Methylthioninium Chloride Enteric-coated Sustained-release Tablets (Lumeblue®) is an oral diagnostic drug, authorized by China’s NMPA for enhancing visualisation of colorectal lesions in grownup sufferers present process screening or surveillance colonoscopy.
- The outcomes of the Section III scientific trial in China present that the Product can considerably enhance the detection price of non-polypoid colorectal lesions (the first endpoint of the research), resulting in an improved detection price of harmful lesions similar to non-polypoid adenomas (the secondary endpoint).
- The Product could be taken through the bowel preparation step, making certain that colorectal staining is accomplished by the point colonoscopy is performed. This not solely enhances the detection price of colorectal lesions but additionally probably simplifies the colonoscopy process, making the examination extra environment friendly and enhancing the screening advantages.
- As of now, CMS’s newly launched progressive portfolio has been expanded to five merchandise, repeatedly producing driving drive to the Group’s sustainable and wholesome growth.
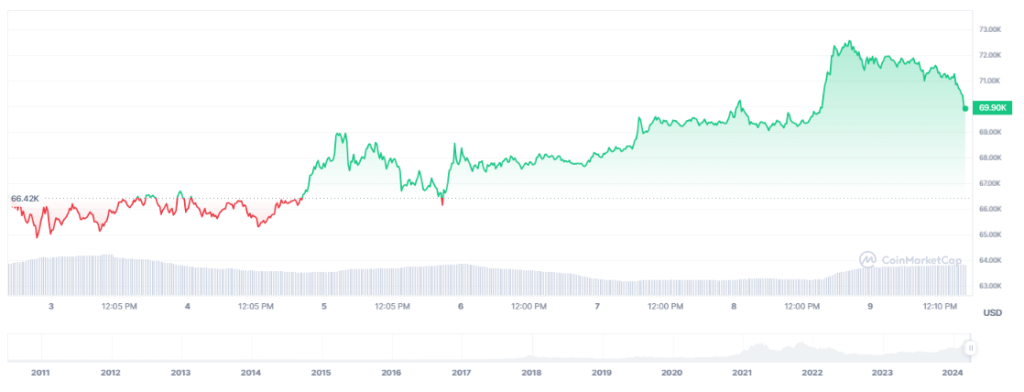
SHENZHEN, CHINA – China Medical System Holdings Restricted (the “Company”, along with its subsidiaries, the “Group” or “CMS”) is happy to announce that on 11 June 2024, the New Drug Utility (NDA) of the Group’s progressive drug methylthioninium chloride enteric-coated sustained-release tablets (Lumeblue®) (the “Product”) has been authorized by the Nationwide Medical Merchandise Administration of China (NMPA). The drug registration certificates was obtained on 18 June 2024. The Product is indicated to enhancing visualisation of colorectal lesions in grownup sufferers present process screening or surveillance colonoscopy, and it’s the first oral methylthioninium chloride enteric-coated sustained-release tablets in China. The Group will synergize with current gastroenterology merchandise and assets to advance the commercialization and tutorial promotion associated works of the Product in an orderly method, aiming to realize nationwide large-scale scientific utility as quickly as doable in order to learn sufferers with colorectal illness.
Lumeblue® is an oral diagnostic drug that makes use of patented multi-matrix (MMX) know-how to ship energetic substances on to the colon and launch them regionally in a managed method. As an enhancer dye, the Product will increase the distinction between colorectal lesions and wholesome mucosa. The outcomes of the Section III scientific trial in China present that the Product can considerably enhance the detection price of non-polypoid colorectal lesions(the first endpoint of the research), resulting in an improved detection price of harmful lesions similar to non-polypoid adenomas (the secondary endpoint)[1]. As well as, the Product could be taken through the bowel preparation step, making certain that colorectal staining is accomplished by the point colonoscopy is performed. This not solely enhances the detection price of colorectal lesions but additionally probably simplifies the colonoscopy process, making the examination extra environment friendly and enhancing the screening advantages.
In line with the analysis and remedy information of the Digestive Endoscopy Department of the Chinese language Medical Affiliation, roughly a complete of 28 million gastroenteroscopies have been accomplished nationwide in 2012, together with 5.83 million colonoscopies. In 2019, roughly 38.73 million gastroenteroscopies have been accomplished nationwide, a rise of 34.62% in contrast with 2012[2]. The Chinese language consensus of early colorectal most cancers screening recommends that individuals aged 50 to 75 years previous must be screened for colorectal most cancers no matter whether or not they have alarm signs[3]. There are roughly 400 million individuals aged 50 to 75 in China in 2020[4]. With the recognition of early screening for colorectal most cancers in China, it’s anticipated to witness a substantial development of the variety of colonoscopies in China sooner or later.
The Product has been authorized by the European Medicines Company (EMA) to be commercialized within the European Union underneath the commerce identify Lumeblue in August 2020. The Group obtained an unique license for the Product from Cosmo Applied sciences Ltd, a totally owned subsidiary of Cosmo Prescription drugs NV, on 3 December 2020.
Pushed by the twin-wheel of “Collaborative R&D and Independent R&D”, CMS has repeatedly deployed world first-in-class (FIC) and best-in-class (BIC) progressive merchandise guided by affected person and scientific calls for. With enhancing R&D capabilities in growing differentiated progressive merchandise, CMS has empowered the continual transformation of scientific analysis outcomes into scientific utility, repeatedly releasing the worth of innovation. As of now, CMS’s newly launched progressive portfolio has been expanded to five merchandise, repeatedly producing driving drive to the Group’s sustainable and wholesome growth. Desidustat Tablets and Methotrexate Injection (rheumatoid arthritis), CMS’s progressive pipeline merchandise, are presently underneath NDA overview in China. In the meantime, over 10 progressive pipeline merchandise are present process scientific trials in China, primarily randomized managed trials (RCT). CMS is predicted to launch differentiated progressive merchandise yearly sooner or later with increased effectivity and extra controllable prices, persevering with to optimize its product portfolio and construct recent driving forces for the mid- to long-term growth of the Firm.
About CMS
CMS is a platform firm linking pharmaceutical innovation and commercialization with robust product lifecycle administration functionality, devoted to offering aggressive services and products to fulfill unmet medical wants.
CMS focuses on the worldwide first-in-class (FIC) and best-in-class (BIC) progressive merchandise, and effectively promotes the scientific analysis, growth and commercialization of progressive merchandise, enabling the continual transformation of scientific analysis into scientific practices to learn sufferers.
CMS deeply engages in a number of specialty therapeutic fields, and has developed confirmed commercialization capabilities, in depth networks and skilled assets, leading to main tutorial and market positions for its main marketed merchandise. CMS continues to advertise the in-depth growth of its advantageous specialty fields and increase enterprise boundaries. Whereas strengthening the competitiveness of the cardio-cerebrovascular/gastroenterology enterprise, CMS independently operates its dermatology and medical aesthetics enterprise, and ophthalmology enterprise, aiming to realize main positions in specialty therapeutic fields, while enhancing the size and effectivity. On the similar time, CMS has expanded its enterprise territory to the Southeast Asian market, striving to turn into a “bridgehead” for world pharmaceutical firms to enter the Southeast Asian market, additional escorting the sustainable and wholesome growth of the Group.
Reference:
- The outcomes of the Section III scientific trial in China was revealed and could be discovered at: https://net.cms.internet.cn/en/2022/12/positive-results-for-china-phase-iii-clinical-trial-of-methylthioninium-chloride-enteric-coated-sustained-release-tablets/
- The analysis and remedy information of the Digestive Endoscopy Department of the Chinese language Medical Affiliation
- Chinese language consensus of early colorectal most cancers screening (2019, Shanghai),Chinese language Journal of Inner Drugs,DOI: 10.3760/cma.j.issn.0578-1426.2019.10.004
- CHINA POPULATION CENSUS YEARBOOK 2020, could be discovered at: https://www.stats.gov.cn/sj/pcsj/rkpc/7rp/zk/indexch.htm
CMS Disclaimer and Ahead-Trying Statements
This press launch isn’t supposed to advertise any merchandise to you and isn’t for promoting functions. This press launch doesn’t suggest any medicine, medical gadgets and/or indications. If you wish to know extra concerning the analysis and remedy of particular illnesses, please observe the opinions or steering of your physician or different medical and well being professionals. Any treatment-related choices made by healthcare professionals must be primarily based on the affected person’s particular circumstances and in accordance with the drug package deal insert.
This press launch which has been ready by CMS doesn’t represent any supply or invitation to buy or subscribe for any securities, and shall not type the premise for or be relied on in reference to any contract or binding dedication in any way. This press launch has been ready by CMS primarily based on data and information which it considers dependable, however CMS makes no illustration or guarantee, categorical or implied, in any way, and no reliance shall be positioned on, the reality, accuracy, completeness, equity and reasonableness of the contents of this press launch. Sure issues mentioned on this press launch could comprise statements relating to the Group’s market alternative and enterprise prospects which might be individually and collectively forward-looking statements. Such forward-looking statements will not be ensures of future efficiency and are topic to identified and unknown dangers, uncertainties and assumptions which might be tough to foretell. Any forward-looking statements and projections made by third events included on this press launch will not be adopted by the Group and the Firm isn’t chargeable for such third-party statements and projections.
Media Contact
Model: China Medical System Holdings Ltd.
Contact: CMS Investor Relations
E mail: ir@cms.internet.cn
Web site: https://net.cms.internet.cn/en/residence/
Supply: China Medical System Holdings Ltd.